Articles from Able Medical Devices

Able Medical Devices, a leading innovator and the first company to introduce a PEEK rigid fixation system for sternal closure, today announced it will showcase its latest sternal closure solutions at the Society of Thoracic Surgeons (STS) 2026 Annual Meeting in New Orleans, Louisiana. The company’s advanced PEEK-based sternal closure technology reflects its ongoing commitment to improving patient outcomes and surgical efficiency.
By Able Medical Devices · Via Business Wire · January 19, 2026

Able Medical Devices has received U.S. FDA 510(k) clearance for its Valkyrie RIB System further enhancing its cardiothoracic and trauma portfolio. The novel single-use, PEEK device is an extension of Able’s Valkyrie Thoracic Fixation System and includes indications for the stabilization and fixation of fractures in the chest wall, reconstructive surgical procedures, trauma, and planned osteotomies.
By Able Medical Devices · Via Business Wire · January 25, 2024
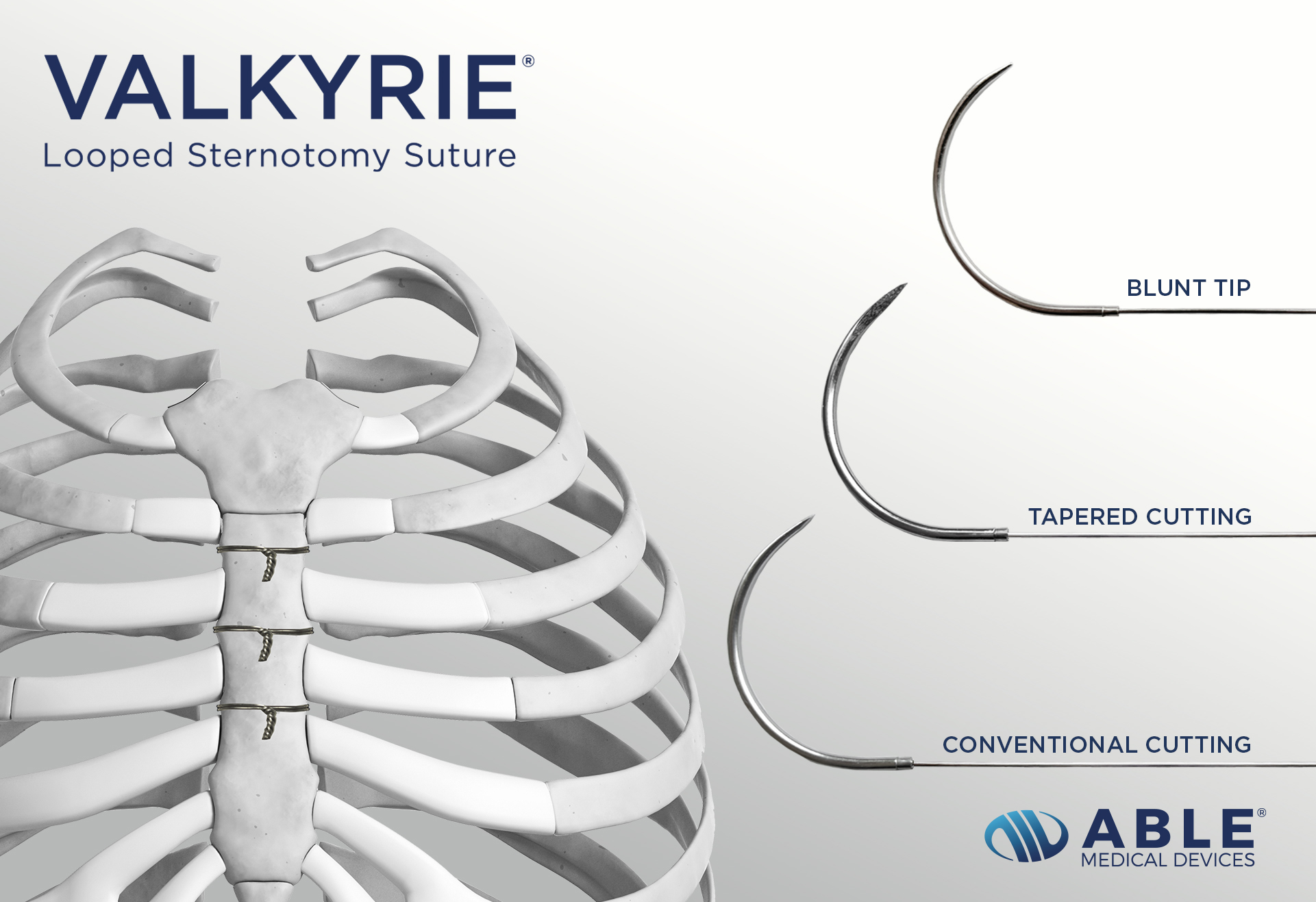
Able Medical Devices today announced the launch of Valkyrie® Looped Sternotomy Sutures.
By Able Medical Devices · Via Business Wire · January 20, 2023

Able Medical Devices announces that the MACH™ Screw Clip System has been granted 510k clearance by the FDA.
By Able Medical Devices · Via Business Wire · August 11, 2021
